January 2, 2025 — The latest announcement on the official website of China’s National Medical Products Administration (NMPA) revealed that the second New Drug Application (NDA) for taletrectinib adipate capsules, a next-generation ROS1 tyrosine kinase inhibitor (TKI) co-developed by AnHeart Therapeutics and Innovent, has been approved. This approval grants the drug’s use as a first-line treatment for ROS1 TKI-naïve adults with locally advanced or metastatic ROS1-positive non-small cell lung cancer (NSCLC).
FAB, as China’s leading medical imaging CRO, provided comprehensive Independent Review Committee (IRC) services for the Phase II clinical study of this drug.
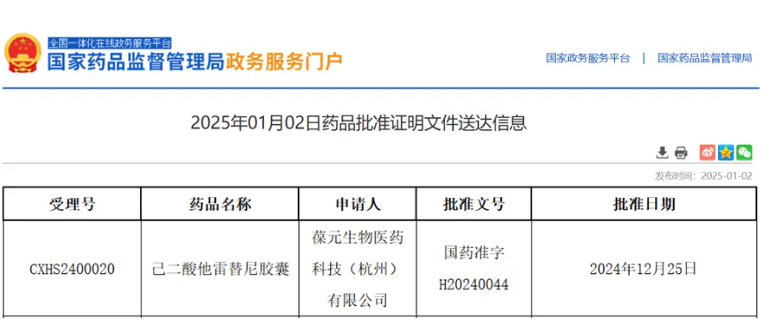
Source: NMPA
Lung cancer ranks among the malignancies with the highest global incidence and mortality rates, with NSCLC accounting for approximately 85% of all lung cancer cases. In China, an estimated 2.6% of NSCLC patients carry the ROS1-positive gene. Furthermore, up to 35% of treatment-naïve metastatic ROS1-positive NSCLC patients present with brain metastases at diagnosis.
Taletrectinib is an oral, potent, brain-penetrant, selective, next-generation ROS1 inhibitor. On December 20, the NMPA granted conditional approval for taletrectinib to treat ROS1-positive NSCLC patients who failed prior ROS1-TKI therapy. This marks the drug’s second approved indication in China.
The approval was based on positive results from the Phase II TRUST-I trial (NCT04395677), a multicenter, open-label, single-arm study conducted in China to evaluate the safety, tolerability, and efficacy of Talbrex® (taletrectinib) in ROS1-positive NSCLC patients. Findings from TRUST-I were published in the Journal of Clinical Oncology (JCO) and presented as an oral presentation at the 2024 ASCO Annual Meeting. Clinical data demonstrated robust and durable antitumor activity with strong intracranial efficacy:
In the treatment-naïve cohort (n=106), the confirmed objective response rate (cORR) assessed by IRC reached 91%, with an intracranial ORR (IC-ORR) of 88%.
At a median follow-up of 23.5 months, the median duration of response (DoR) and progression-free survival (PFS) assessed by IRC were not yet reached.
Source: Innovent
Since 2019, FAB has served as the third-party independent imaging evaluator for the pivotal clinical studies supporting this approval. Leveraging deep domain expertise, senior expert teams, and a highly efficient project management system, FAB ensured timely, high-quality delivery of results, significantly contributing to the drug's successful approval.
Designing protocol-compliant evaluation systems to meet trial-specific requirements.Implementing a proprietary electronic integrated management platform to enhance assessment efficiency and accuracy, while ensuring data integrity for endpoint reliability.Maintaining seamless communication with sponsors and clinical sites, providing instant professional feedback on imaging-related inquiries.Rigorous adherence to timelines for image reviews, achieved through advanced planning and expert coordination.
Following the successful NMPA inspection, AnHeart Therapeutics expressed profound gratitude to FAB for its rigorous professionalism and exceptional service quality, highlighting the critical role of their collaboration.
To date, FAB has supported 32 approved drug projects (including 8 in the NSCLC field) and facilitated 53 NDA submissions, with 42 projects passing regulatory inspections. These milestones underscore FAB's industry-leading expertise in independent imaging evaluation and its unmatched execution capabilities.
FAB extends heartfelt congratulations to its partners on this milestone and remains committed to accelerating the development of innovative therapies and medical devices, bringing new hope to patients worldwide.












 Home
Home Services
Services Telephone
Telephone Message
Message