On March 10, 2025, the NMPA approved a new indication for Kelun-Biotech’s TROP2-targeting antibody-drug conjugate (ADC) Sacituzumab Tirumotecan for Injection. It is indicated for adult patients with locally advanced or metastatic EGFR-mutated NSCLC who failed prior EGFR-TKI and platinum-based chemotherapy.
FAB, as China’s leading medical imaging CRO, provided comprehensive Independent Review Committee (IRC) services for the Phase II/III clinical study of this drug.
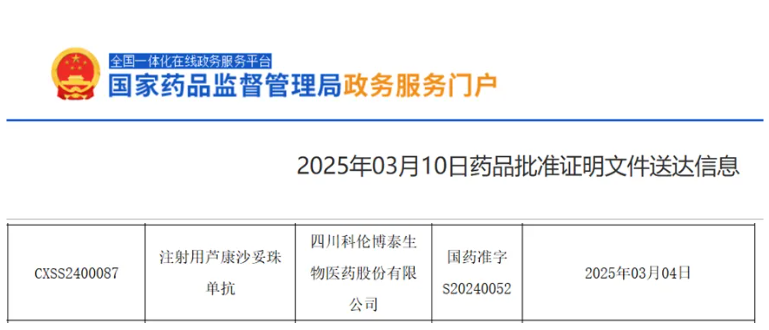
Source: NMPA
This approval was based on the pivotal multicenter, randomized, controlled OptiTROP-Lung03 study, which evaluated the efficacy and safety of sac-TMT monotherapy (5mg/kg IV Q2W) versus docetaxel in locally advanced or metastatic EGFR-mutated NSCLC patients after failure of EGFR-TKI and platinum-based chemotherapy (sequential or combined). Prespecified interim analysis demonstrated that sac-TMT monotherapy showed statistically significant and clinically meaningful improvements in objective response rate (ORR), progression-free survival (PFS), and overall survival (OS) compared to docetaxel.
This marks the first innovative ADC globally to demonstrate statistically and clinically significant OS benefits versus standard therapy in EGFR-mutated NSCLC patients post-EGFR-TKI and platinum-based chemotherapy failure.
Source: Kelun-Biotech
As a key partner in Kelun-Biotech’s Phase II and III clinical studies, FAB undertook the critical responsibility for independent central imaging review. This collaboration represents FAB’s fifth milestone project delivery this year.
The Phase II/III studies enrolled over 500 subjects. Leveraging extensive expertise in lung cancer trials, a senior image review team, and efficient operational execution, FAB provided pivotal support for high-quality endpoint data delivery and smooth regulatory review, accelerating patient access to this novel ADC.
Quality control is paramount in medical imaging assessment. FAB implemented:
100% imaging specialist-led QC
Efficient issue communication protocols
Real-time medical imaging consultation
Ensuring data accuracy, consistency, and prompt resolution of technical queries.
According to Ziji Li, Associate Director of Clinical Operations: "During the data cutoff (DCO) phase, our team entered 'combat mode' – overcoming tight timelines to complete all reviews and deliver high-quality results on schedule. This not only ensured trial progression but secured crucial time for regulatory review."
Beyond central review, FAB provided on-site support during the NMPA inspection. The team demonstrated exceptional project familiarity and professionalism, addressing inspector queries with precision and achieving zero imaging-related findings, earning high client praise.
FAB looks forward to partnering with more innovators, delivering premium independent central review services to accelerate novel drug development and expand treatment options for patients worldwide.












 Home
Home Services
Services Telephone
Telephone Message
Message