On March 10, 2025, China's NMPA approved a new indication for Hansoh Pharma’s Almonertinib Mesilate Tablets (Ameile®) for maintenance therapy in unresectable, locally advanced EGFR-mutant NSCLC patients without progression post platinum-based chemoradiotherapy.
FAB, as China’s leading medical imaging CRO, provided Independent Review Committee (IRC) services for the Phase III clinical study of this drug.
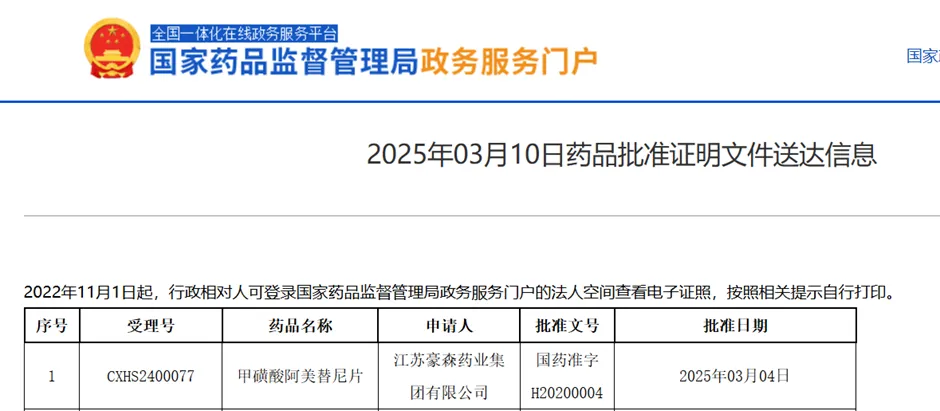
Source: NMPA
The approval was primarily based on the POLESTAR study (HS-10296-304), a randomized, double-blind, placebo-controlled, multicenter phase III clinical trial to evaluate the effects of Ameile as maintenance therapy in unresectable stage III NSCLC following chemoradiotherapy.
The study results showed that Ameile reduced the risk of disease progression by more than 80% and the median progression-free survival (mPFS) for patients treated with Ameile was 30.4 months compared with just 3.8 months for those receiving placebo. Additionally, the benefit of Ameile over placebo for PFS were consistent across all predefined subgroups, indicating a comprehensive benefit profile. Furthermore, the objective response rate (ORR) in the Ameile treatment group assessed by BICR reached 57%, with the median duration of response (DoR) extending to 16.59 months and median overall survival (OS) not yet reached. The incidence of CNS lesions and distant metastases were lower. The overall tolerability and manageability of Ameile in patients after chemoradiotherapy were favorable. In terms of adverse events (AEs), the incidence of≥3 grade radiation pneumonitis was 0, and the incidence of interstitial pneumonia was also 0.
Source: Hansoh Pharma
As a long-standing strategic partner of Hansoh Pharma, FAB was entrusted with the pivotal responsibility for independent central review in this critical study. Leveraging deep expertise and operational excellence, we safeguarded imaging data quality throughout the trial lifecycle, optimized resource coordination, and delivered high-quality review outcomes on schedule—directly contributing to the new indication's approval.
FAB provides sponsors with an integrated end-to-end solution leveraging its independently developed lung cancer imaging evaluation system. This solution encompasses the entire workflow, from site data upload and centralized image quality control to electronic image review and post-review medical adjudication. The system has undergone rigorous validation and features comprehensive audit trails, ensuring data integrity and traceability.
During critical phases of the Ameile project, the FAB team demonstrated exceptional commitment. Maintaining a high-efficiency workflow—from midday QC checks to late-night review monitoring—the team consistently met urgent deadlines. To ensure project progression, they established a *24-hour response mechanism*, enabling rapid QC completion post-upload and efficient resolution of queries. Additionally, the team proactively developed review timelines, coordinated expert schedules, and adhered to scientific rigor to complete all evaluations on schedule.
Through this approach, the project achieved a key outcome: IRC-assessed drug efficacy exceeded the expected target, providing critical support for the product’s successful regulatory approval.
It is noteworthy that FAB provided comprehensive third-party independent imaging evaluation services for the first two approved indications of Almonertinib Mesylate Tablets (Ameile®). From second-line to first-line therapy, and further to maintenance therapy, FAB has played a pivotal role across multiple Ameile® clinical studies. This consistently demonstrates the sponsor's high recognition of and sustained trust in FAB's professional expertise and project quality.
Three Indications Timeline
Approval | Indication |
Mar 2020 | Second-Line Treatment T790M+ NSCLC |
Dec 2021 | First-Line Treatment EGFRm NSCLC |
Mar 2025 | Maintenance post-chemoradiation |
FAB congratulates Hansoh Pharma on this strategic achievement!












 Home
Home Services
Services Telephone
Telephone Message
Message