On December 23, 2024, China’s NMPA officially approved the new drug application of Stapokibart Injection, a Class 1 innovative biologic developed by KeyMed Biosciences, for the treatment of chronic rhinosinusitis with nasal polyps. The indication had previously been granted priority review.
FAB, as China’s leading medical imaging CRO, provided Independent Review Committee (IRC) services for the Phase III clinical study of this drug.
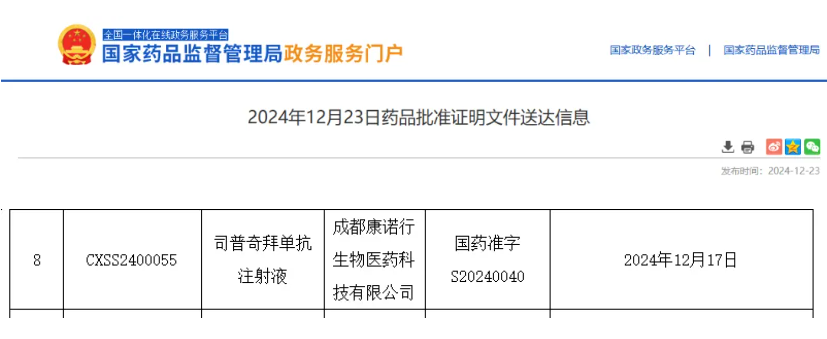
Source: NMPA
Chronic rhinosinusitis with nasal polyps is a highly challenging clinical condition. Symptoms such as nasal congestion, difficulty breathing, and reduced sense of smell significantly impact patients’ quality of life. Traditional treatments, including pharmacotherapy and endoscopic sinus surgery, often face limitations such as suboptimal efficacy, short duration of effect, and disease recurrence. There is a pressing need for innovative therapies. The approval of Stapokibart for this indication provides a new treatment option for patients in China.
Stapokibart (CM310) is a highly potent humanized antibody targeting IL-4Rα. It mediates dual blockade of the signaling pathways of IL-4 and IL-13, two key cytokines driving type 2 inflammation. On September 12 this year, Stapokibart was first approved in China for the treatment of moderate-to-severe atopic dermatitis in adults, making it the first IL-4Rα antibody drug approved in China and the second globally.
This latest approval was based on a multicenter, randomized, double-blind, placebo-controlled Phase III clinical study designed to confirm the efficacy and safety of Stapokibart in patients with chronic rhinosinusitis with nasal polyps. The study enrolled 180 subjects, randomized in a 1:1 ratio to receive either Stapokibart 300 mg or placebo every two weeks, for a total of 12 doses during the double-blind period. The co-primary endpoints were the change from baseline in nasal polyp score (NPS) and nasal congestion score (NCS) at week 24.
The results showed positive outcomes for the Phase III trial. Both co-primary endpoints were met with highly statistically significant differences (all p-values < 0.0001) favoring the Stapokibart group over the placebo group, alongside a favorable safety profile.

Source: KeyMed Biosciences
Since 2022, FAB and KeyMed Biosciences have established a strong collaborative partnership. FAB played an integral role in the central imaging assessment for the Phase III clinical study. Throughout the project lifecycle, FAB leveraged its extensive efficacy evaluation experience, efficient project team, and robust management system to rapidly respond to the sponsor’s needs, contributing to the successful approval of the product.
The trial utilized a combination of nasal endoscopy and sinus CT for efficacy evaluation, presenting new challenges due to the complexity and variability of endoscopic images and the high timeliness requirements for quality control. In response, the FAB team proactively addressed these challenges through close collaboration with all stakeholders, ensuring smooth project progression. The team completed the image review for the primary endpoints with high quality two weeks ahead of the sponsor’s deadline, demonstrating exceptional project execution capabilities.
Stringent control over baseline enrollment was critical to the project's success. FAB took on the crucial role of enrollment assessment gatekeeper, rigorously screening over 360 subjects within just 10 months. The team completed quality control within 15 minutes of receiving endoscopy data uploaded by study sites. This efficient QC process laid the foundation for accurate assessment data. "Any delay could potentially affect subject enrollment and even require re-recording of endoscopy videos." Facing such requirements and challenges, the FAB team demonstrated tremendous perseverance and a high sense of responsibility, working tirelessly day and night to keep the project on schedule.
Data quality control was just the beginning; the subsequent image review posed even greater challenges. All screening period assessments for subjects were completed within two calendar days to determine eligibility. This rapid and accurate process was made possible by the full commitment of every FAB team member and review expert, enabling high-quality subject enrollment within a short timeframe.
Throughout the double-blind treatment period, the project team maintained close collaboration with the sponsor, ensuring all activities adhered to the established timeline. Nearly 2,000 timepoints were reviewed within just six months, an achievement that earned high praise and recognition from the sponsor. The sponsor expressed great satisfaction with FAB's high-quality deliverables, laying a solid foundation for future collaboration.
It is worth highlighting that FAB has achieved a new record with 31 projects successfully approved to date! Furthermore, we have provided professional IRC services to over a hundred domestic and international companies. While maintaining deep expertise in oncology, FAB has also expanded extensively into non-oncology areas, covering key therapeutic fields such as respiratory, nervous system, digestive system, cardiovascular, dermatology, orthopedics, and ophthalmology. With over 300 projects executed, 53 NDAs submitted, and 42 projects successfully passing inspections, FAB continues to demonstrate excellent delivery capabilities in providing high-quality and efficient services.
FAB remains committed to the relentless pursuit of excellence, striving to be the most trusted long-term partner for sponsors. We look forward to continuing our collaboration on the path of pharmaceutical innovation, achieving even greater milestones together!












 Home
Home Services
Services Telephone
Telephone Message
Message