On February 7, 2024, the official website of China’s National Medical Products Administration (NMPA) announced the approval of the marketing application for Kelun-Biotech’s self-developed recombinant epidermal growth factor receptor (EGFR) human-mouse chimeric monoclonal antibody (mAb) Cetuximab N01 Injection (formerly A140). This product is approved for use in combination with FOLFOX or FOLFIRI regimens as first-line treatment for metastatic colorectal cancer with RAS wild-type genes, at a dose of 500 mg/m² every two weeks, or with a starting dose of 400 mg/m² and a maintenance dose of 250 mg/m² weekly. FAB, as China’s leading medical imaging CRO, provided Independent Review Committee (IRC) services for the Phase III clinical study of this drug.
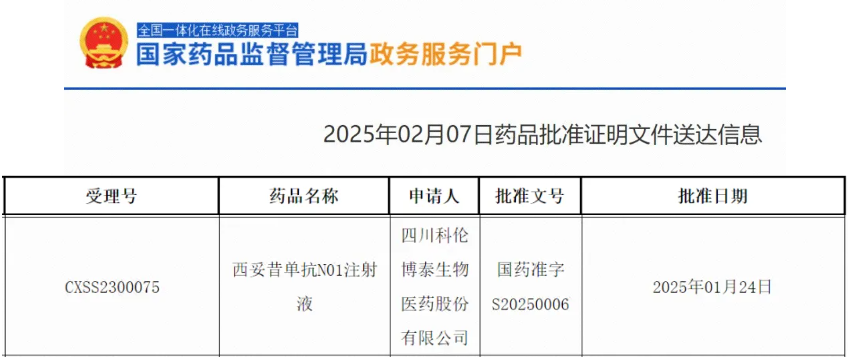
Source: NMPA
The approval of Cetuximab N01 Injection was based on a randomized, double-blind controlled, multicenter Phase III clinical study. This is the first large-sample Phase III clinical trial in China to conduct a head-to-head comparison with Cetuximab Injection (Erbitux®) combined with chemotherapy for the first-line treatment of patients with RAS wild-type metastatic colorectal cancer (enrolling 688 subjects). Clinical data showed that the Cetuximab N01 combination chemotherapy regimen demonstrated clinical equivalence in objective response rate (ORR) compared to Cetuximab Injection (Erbitux®) combined with chemotherapy (Cetuximab N01 vs. Cetuximab Injection [Erbitux®]: 71.0% vs. 77.5%; ORR ratio: 0.93 [95% CI: 0.87, 0.99]). For duration of response (DoR) and progression-free survival (PFS), no clinically or statistically significant differences were observed between Cetuximab N01 and Cetuximab Injection (Erbitux®) (median PFS: 10.9 months vs. 10.8 months, HR: 1.03 [95% CI: 0.83, 1.28]; median DoR: 10.2 months vs. 9.5 months). In terms of safety, this large-sample Phase III head-to-head clinical trial fully confirmed that the Cetuximab N01 combination chemotherapy regimen had comparable safety, tolerability, and immunogenicity to Cetuximab Injection (Erbitux®) combined with chemotherapy.

Source: Kelun-Biotech
As a long-term partner of Kelun-Biotech, FAB comprehensively undertook the core task of independent central imaging review (IRC) for the Phase III clinical study of Cetuximab N01 Injection. Leveraging over a decade of professional expertise and project experience in the medical imaging field, FAB ensured the high-quality progression of the evaluation process through its senior expert team, efficient and rigorous project management system, and medical support capabilities. This provided critical support for the scientific validity and credibility of the clinical data for Cetuximab N01 Injection, facilitating its successful regulatory approval.
FAB designed and developed a compliant imaging evaluation system tailored to the specificities of colorectal cancer. Its self-developed end-to-end electronic management system covers the entire chain of image acquisition, transmission, anonymization, blinded review, and data storage, enabling intelligent and integrated management of the evaluation process. This system not only significantly enhances the efficiency of efficacy assessment but also ensures the high reliability of study endpoints through data integrity and traceability, solidifying the technical foundation for the objectivity of clinical conclusions.
During the enrollment phase of this project, FAB provided baseline imaging eligibility assessment services, ultimately successfully enrolling 688 subjects. Facing the high complexity of 81 multicenter studies, FAB established a 360° seamless communication mechanism, maintaining close contact with research centers during project initiation, training, and imaging data upload management. Simultaneously, the team engaged in frequent interactions with the sponsor regarding review progress management to ensure efficient project advancement. To meet the stringent timelines for image reviews, team members sacrificed personal time, responded promptly to project demands, and actively coordinated expert schedules to ensure each review proceeded smoothly as planned. As a result, Kelun-Biotech repeatedly commended FAB’s team for their meticulous professional contributions and explicitly expressed their intention to deepen strategic collaboration.
The approval of Cetuximab N01 Injection marks another achievement for FAB in the field of oncology. To date, FAB has supported the successful approval of 33 projects and submitted 54 NDA applications, 43 of which have successfully passed inspection. Every milestone is etched with the relentless efforts of the FAB team, who work tirelessly day and night.
FAB looks forward to collaborating with more partners to provide independent central imaging evaluation services, accelerating the global innovation drug development process and enabling more effective treatments to benefit more patients. As Dr. Luxia Liang, founder of FAB, stated, “Behind every piece of imaging data lies the weight of life. We approach every step of drug development with reverence and dedication.”












 Home
Home Services
Services Telephone
Telephone Message
Message