On February 28, 2025, China's National Medical Products Administration (NMPA) officially announced the approval of the marketing application for Finotonlimab Injection (Anyouping®, SCT-I10A) - a recombinant humanized anti-PD-1 IgG4 monoclonal antibody independently developed by Sinocelltech - in combination with Bevacizumab Injection. This therapy is approved for the first-line treatment of patients with unresectable or metastatic hepatocellular carcinoma (HCC) who have not received prior systemic treatment.
FAB, as China’s leading medical imaging CRO, provided Independent Review Committee (IRC) services for the Phase III clinical study of this drug.
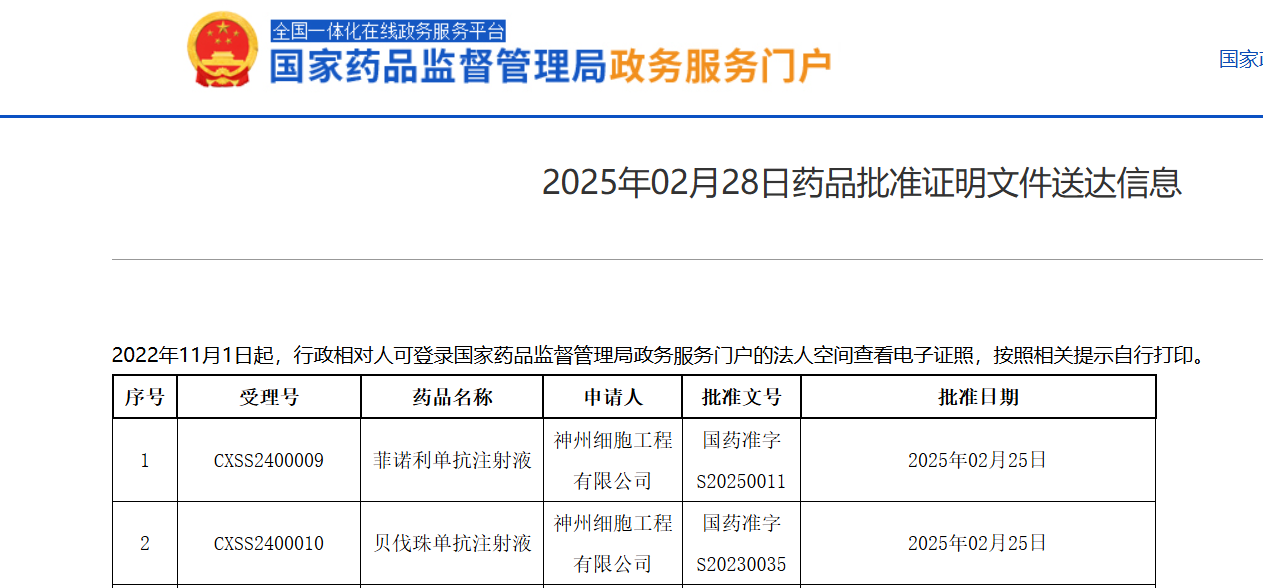
Source: NMPA
Finotonlimab (SCT-I10A) is a recombinant humanized anti-PD-1 IgG4 monoclonal antibody independently developed by Sinocelltech. It was previously approved in China in combination with chemotherapy for the treatment of head and neck squamous cell carcinoma, making it the first domestically developed PD-1 monoclonal antibody approved for this indication.
SCT510 is a recombinant humanized anti-VEGF monoclonal antibody injection developed by Sinocelltech, serving as a biosimilar to Bevacizumab Injection (Avastin). Previously, Bevacizumab has received NMPA approval for treating multiple indications including metastatic colorectal cancer, advanced/metastatic or recurrent non-small cell lung cancer, recurrent glioblastoma, hepatocellular carcinoma, cervical cancer, as well as epithelial ovarian cancer, fallopian tube cancer, or primary peritoneal cancer.
This approval is based on a multicenter, randomized, open-label Phase II/III clinical study evaluating SCT-I10A combined with SCT510 versus sorafenib as first-line treatment for advanced hepatocellular carcinoma. Clinical data showed that the combination of Finotonlimab and Bevacizumab (SCT510) developed by Sinocelltech achieved remarkable results in clinical studies. Particularly, the Phase III study met both the median progression-free survival (mPFS) and median overall survival (mOS) dual endpoints (Finotonlimab vs. sorafenib: mPFS 7.1 months vs. 2.9 months [HR=0.50, P<0.0001]; mOS 22.1 months vs. 14.2 months [HR=0.60, P=0.0008]), while also achieving an objective response rate of 32.8%.

Source: Sinocelltech
As a long-term partner of Sinocelltech, FAB comprehensively undertook the core task of independent central imaging review (IRC) for the Phase III clinical study of this Finotonlimab and Bevacizumab (SCT510) combination therapy.
Facing a complex project involving 346 subjects across 67 research centers, FAB leveraged its extensive efficacy evaluation experience, team of senior imaging interpretation experts, and highly efficient project execution team to provide crucial support for the high-quality delivery of clinical endpoint data and the smooth passage of regulatory review, helping to accelerate the availability of this combination therapy for more hepatocellular carcinoma patients.
Throughout the entire process from project initiation to successful closure, FAB maintained high-frequency communication and close coordination with all parties. According to the specific requirements and evaluation criteria of the trial, FAB provided systematic and comprehensive training for readers. In the critical aspect of image quality control management, the FAB team established strict quality standards and processes to ensure the accuracy and consistency of imaging data. When image quality issues were identified, immediate feedback was provided and quickly resolved through efficient communication. Additionally, the FAB team responded to various medical imaging consultations in real-time mode, covering not only professional central imaging consultations for study protocols but also providing professional guidance and answers during the image review process.
A liver cancer imaging evaluation system was designed and developed according to efficacy endpoint assessment requirements. This system comprehensively covered every aspect of image transmission, anonymization, blinded review, and data storage, significantly improving the efficiency of efficacy evaluation while providing strong technical support for the integrity and traceability of clinical data.
"There are no shortcuts to professionalism, only extreme control over every piece of imaging data and full commitment to every promise," said project manager Zhou Kun. Under her leadership, the project team maintained a high sense of responsibility and mission. To meet unexpected review demands, team members sacrificed rest time, working through two consecutive weekends to complete tasks, ensuring that each review strictly followed timelines, receiving praise from the sponsor.
The successful approval of this combination therapy marks another milestone for FAB in the field of liver cancer. Previously, the company had supported the approval of donafenib tosilate tablets for first-line treatment of advanced hepatocellular carcinoma and icaritin soft capsules for first-line treatment of advanced hepatocellular carcinoma with poor baseline prognosis. To date, FAB has provided central imaging IRC services for more than 320 clinical trials, supported the successful approval of 34 projects, and submitted 56 NDA applications.
FAB looks forward to collaborating with more partners to provide higher-quality independent central imaging evaluation services, support innovative drug research and development, and bring new treatment options to more patients!












 Home
Home Services
Services Telephone
Telephone Message
Message